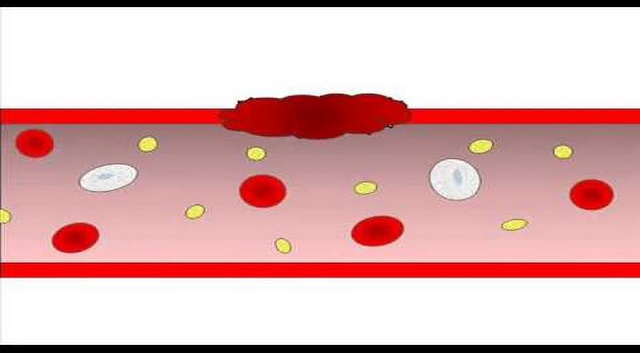
Platelets are discoid blood components without a nucleus, varying between 2-3 microns in diameter. It circulates in the blood for 7-10 days before being disposed by the macrophages.
Press Ctrl+D to bookmark this page. You might need it in the future.
- READ MORE